
Improving Outcomes for Proned Patients in Intensive Care
IMPROVING PRONE REPOSITIONING
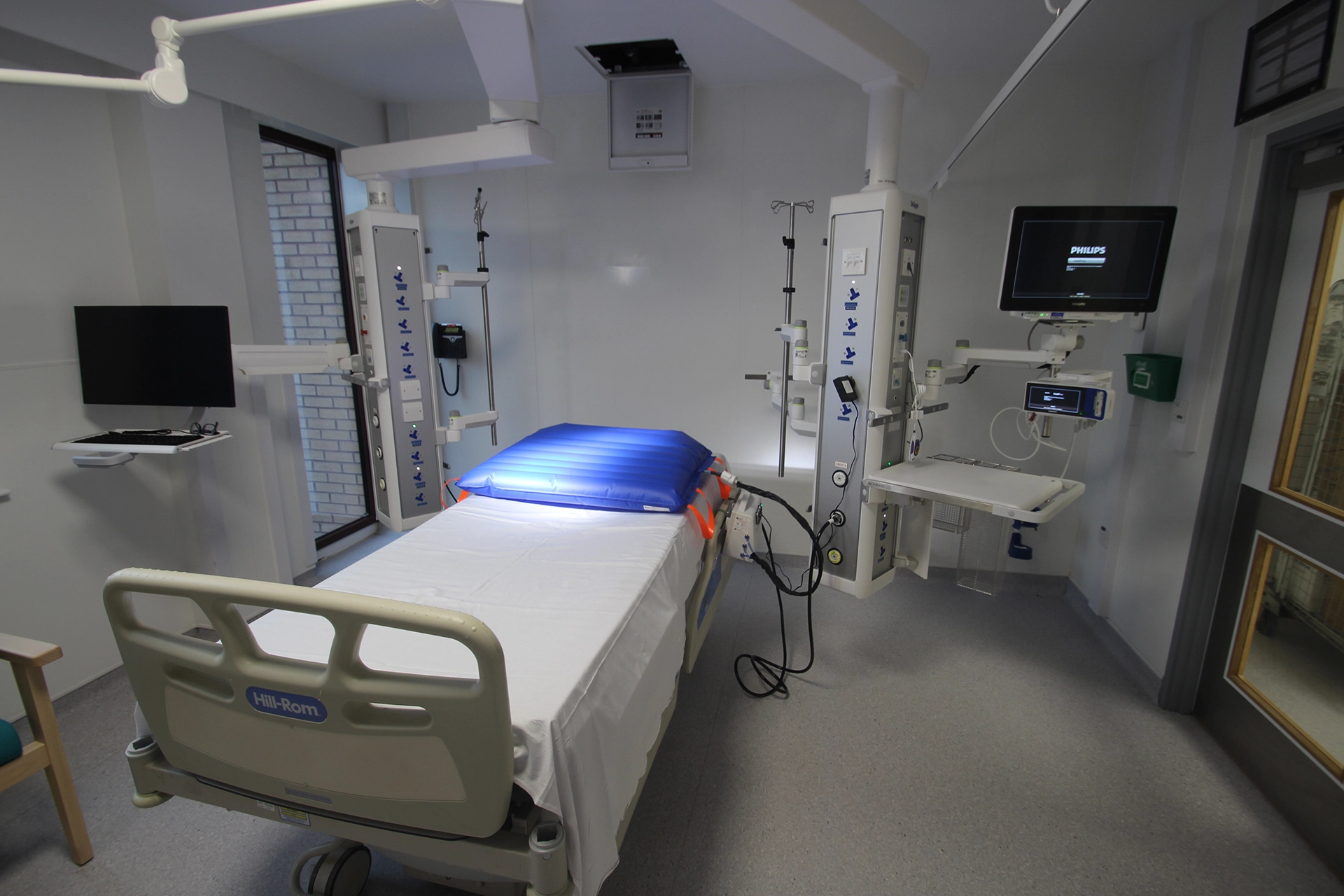
What is the BathMat?
The BathMat is an inflatable repositioning device, created to help doctors and nurses safely move critically ill patients.
Previously this has been a complicated, manual task that uses sliding sheets or hoists. Over 16,500 patients are cared for in this way in the NHS each year, accounting for over half a million staff hours.
The BathMat is a novel medical device that simplifies patient repositioning via the use of a controllable inflatable vessel which makes repositioning safer for patients and staff.
Improving critical care
What is the aim of the BathMat?
The ‘BathMat’ inflatable prone repositioning device aims to make care safer for proned patients on Intensive Care. We are performing our first-in-patient trial to investigate the following factors.
Improvements to safety
Repositioning involves moving patients to reduce the likelihood of pressure sores. The BathMat Increases the speed and reduces amount of physical movement required to safely perform this exercise, aiming to greatly reduce the likelihood of injury for both staff and patients.
Reduced burden on staff
Regular repositioning prevents pressure sores, but current methods require 5-7 staff for over 30 minutes – a challenge in busy ICUs. The BathMat enables repositioning with just 2 people in under 10 minutes, allowing for more frequent repositioning and freeing up staff for other important care duties.
Reduces sores and injury
An ability to more easily and frequently perform repositioning has the potential to reduce the likelihood of patients generating pressure sores. The BathMat therefore aims to improve patient outcomes by reducing the likelihood of this type of complication which may arise from being placed in the prone position.
Have some questions?
Everything you need to know
Have a question about your the BathMat? Take a look at our most frequently asked questions.
What is proning?
Proning is a way of helping people who are very sick and have trouble breathing. It involves lying patients on their front to get more oxygen into their body. When in this position, doctors need to turn the patients’ head and move their arms every 2-4 hours. Doctors call this repositioning. It helps prevent sores as well as other injuries. This is currently performed by a team of 5+ staff and takes lots of time and resources. The process can also be dangerous because it requires a lot of movement which can hurt patients and staff.
How are people recruited to the trial?
Because our patients will be on Intensive Care as an emergency, we are only able to ask patients if they want to take part when they wake up. We will speak to a member of their family or an independent doctor to see if they think they would be happy to be involved, and speak to patients once they have recovered.
How will the results be used?
To share the results with other doctors, we will write reports and give presentations. If successful, we will start making and using the device to help us care for our patients. Everyone involved in this study will keep your data safe and secure. We will follow all privacy rules. At the end of the study, we will save some of the data in case we need to check it and to write scientific reports. We will make sure no-one can work out who is involved from the reports we write.
What are the changes to care associated with being included?
Patients in the trial will experience the same quality of care as those who did not participate. The only difference is that patients in the trial will be periodically repositioned using the BathMat, rather than the use of prior manual methods. Staff then record the time it took, number of staff required, any delays to care, and any complications due to proning.
What happens when the research study stops?
Once we have recruited a sufficient number of patients, we will assess the data we have collected to see if we can prove our device makes any difference to patient care. The results will be used in order to make a published report in a scientific journal.
Who is organising and funding this study?
The study is being organised by a team working at the Royal United Hospitals Bath NHS FT, working closely with a team at the University of Bath. It is funded by the National Institute of Health Research.
Who has reviewed the research?
The research study has been independently reviewed as part of our application for funding from the National Institute of Health Research. All research in the NHS is looked at by an independent group of people, called a Research Ethics Committee, to protect your interests. This study has been reviewed and given favourable opinion by the TBC Ethics Committee and the MHRA.
What will happen to the results of the study?
The fully anonymised results of this study will be presented at academic meetings nationally and internationally. They will be used to provide information to allow us to demonstrate the benefits of the BathMat for our patients and staff so we can continue to use it.
Who do I contact for further information?
If you have any questions, please contact us using the form at the bottom of this webpage.
Have some questions?
Get in touch
Use the form below to connect with us to discuss your specific needs and explore how we can assist you.
Would you like to get involved in a trial?
We are actively searching for patient and public involvement in the development of our device. If you would be interested in finding out more, please get in touch via this form. Your contribution will help ensure we maximise the potential of the device, and ensure the approach meets the needs of all stakeholders.